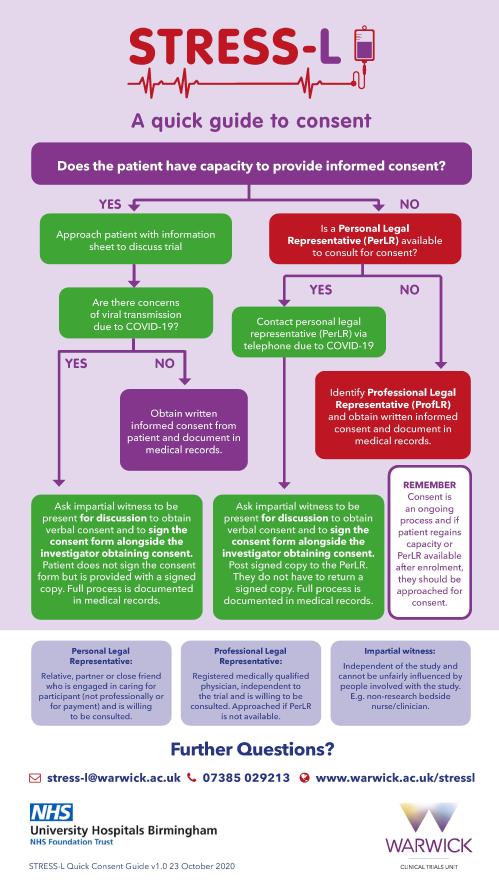
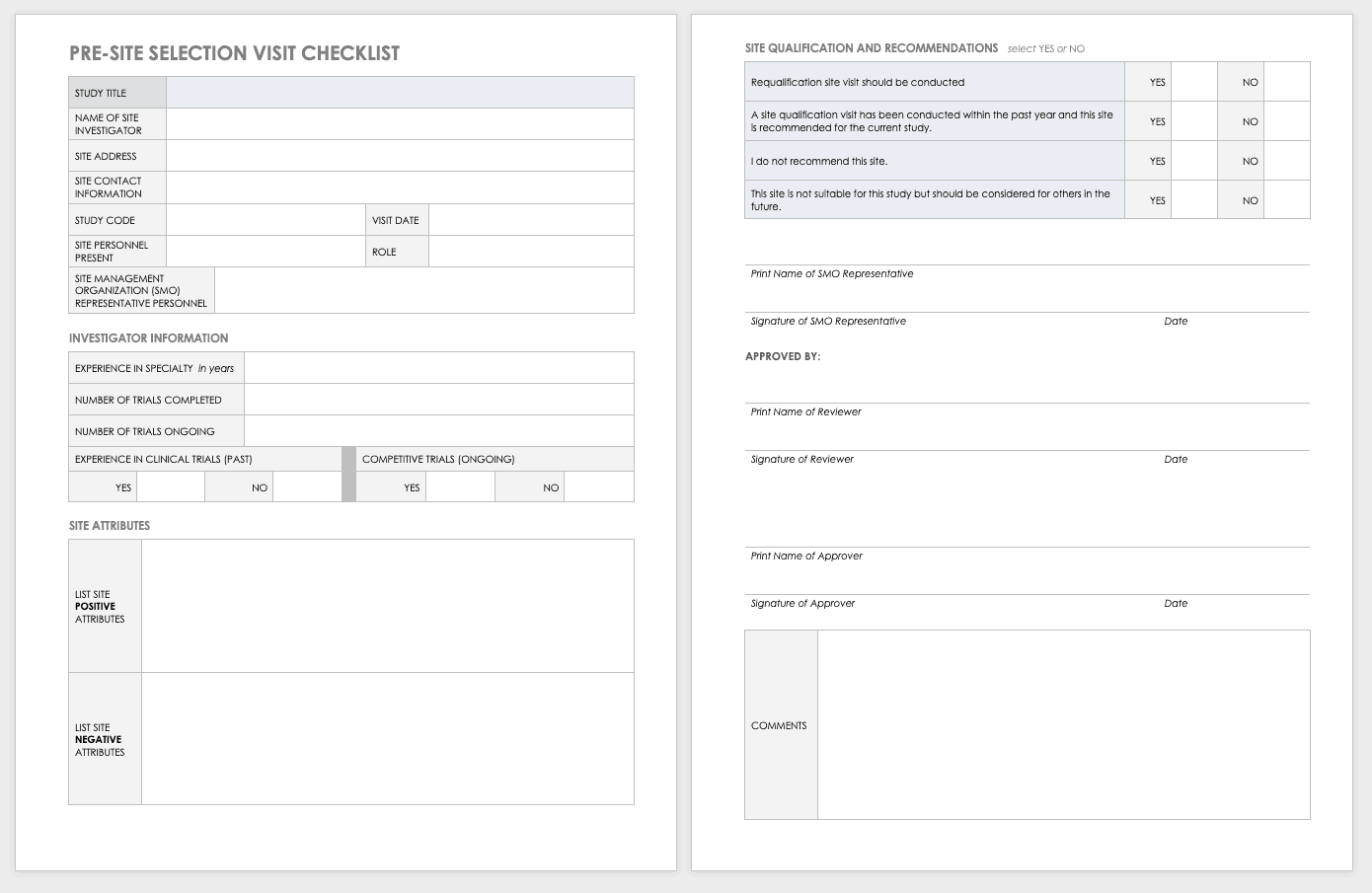
PLOS ONE: Differences in Investigator-Initiated Trials between Japan and Other Countries: Analyses of Clinical Trials Sponsored by Academia and Government in the ClinicalTrials.gov Registry and in the Three Japanese Registries

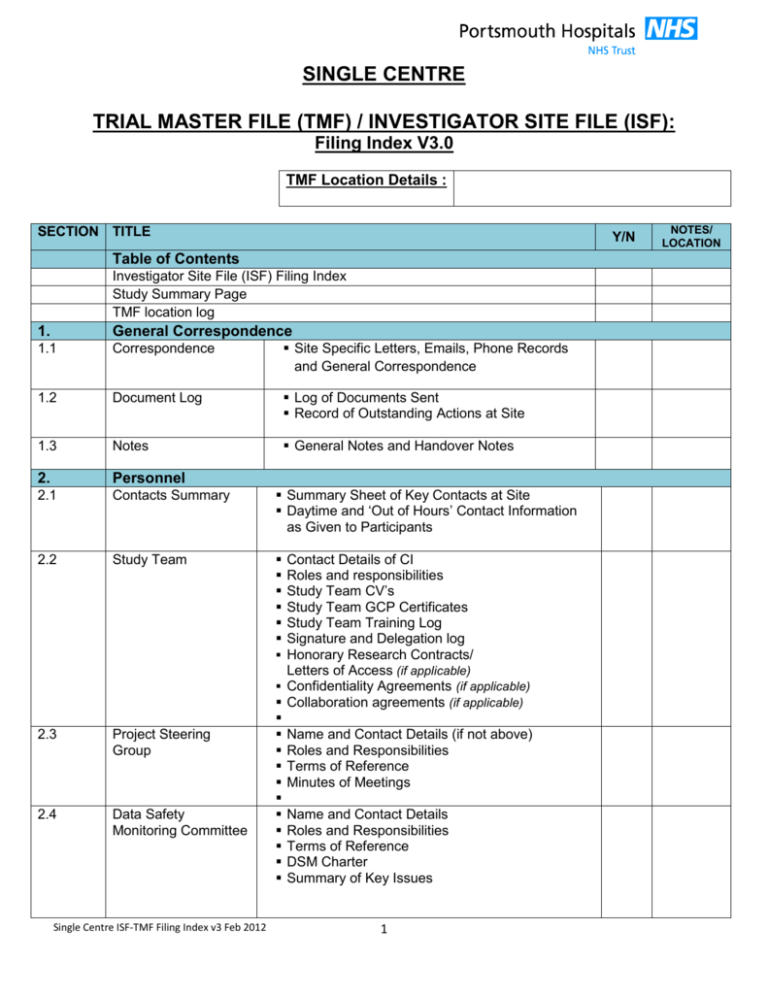
ICH GCP — Clinical Research Blog | Certified Clinical Research Professionals Society - Clinical Research Certification